ABSTRACT
All over the world the presence of arsenic in water sources for human consumption has been raising great concern in terms of public health since many epidemiologic studies confirm the potential carcinogenic effect of arsenic. Because arsenic removal is the most frequent option for safe drinking water, the development of more efficient and sustainable technologies is extremely important.
Membrane separation processes are suitable for water treatment because they can provide an absolute barrier for bacteria and viruses, besides removing turbidity and colour. Their application is a promising technology in arsenic removal since it does not require the addition of chemical reagents nor the preliminary oxidation of arsenite required in conventional treatment options. However, since membrane technologies such as reverse osmosis can be a very expensive and unsustainable treatment option for small water supply systems, it becomes crucial that alternative methods are developed.
This work presents a few conclusions based on a laboratorial study performed to evaluate the efficiency of arsenic removal using ultrafiltration, microfiltration and solar oxidation processes under different experimental conditions for relevant parameters. The results showed removal efficiencies higher than 90%.
1. INTRODUCTION
The presence of arsenic in natural waters can occur naturally or result from anthropogenic activities, generally emerging with higher concentrations in groundwater. The contamination of drinking water sources has a wide variety of adverse health effects, including severe skin lesions (Fig. 1), cardiovascular and haematological effects, and neurological disturbances effects have been attributed to chronic arsenic exposure (Yoshida et al., 2004).
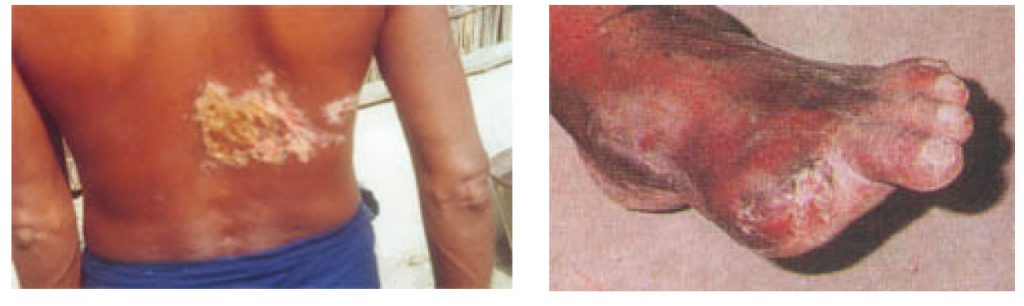
(http://www.sos-arsenic.net/english/contamin/index.html)
Acute and sub acute poisoning results from ingestion of large quantities of arsenic with lower exposure time, whereas chronic poisoning occurs due to consumption of arsenic contaminated water for a long time period. In 1993, the World Health Organization (WHO) had decreased the maximum contaminant level (MCL) standard of arsenic in drinking water from 50 to 10 ppb.
In this study, advanced filtration processes were analyzed using two different membranes for microfiltration (MF) and ultrafiltration (UF) processes designed to separate solute and solvent components in the solution using pressure as driving force. In both cases, their behaviour is similar as the solvent (water) flows through the membrane and the solutes are rejected by the action of a pressure gradient. Compared to ultrafiltration, the microfiltration process has its main advantage on the significant reduction in operating costs (energy and filter material).
The aim of this study is to evaluate the efficiencies of microfiltration and ultrafiltration processes, as emerging technologies for arsenic removal in public water supply systems, at a laboratorial scale. The effects of operating conditions on membranes performance, like pressure gradient, initial arsenic concentration, temperature, pH and Fe:As ratio in efficiency results were studied.
2. METHODS
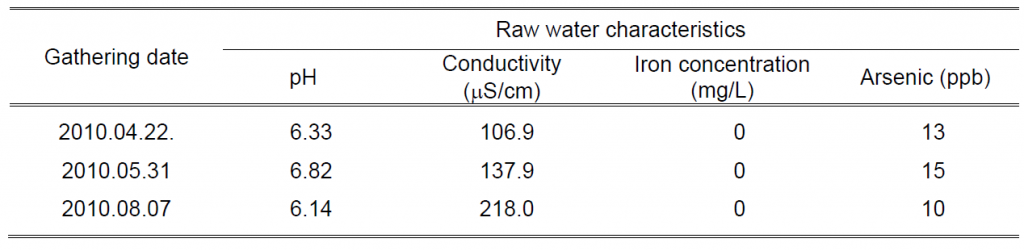
The raw water containing arsenic used in the laboratory tests was collected in a groundwater abstraction point located in Fafe, belonging to the Águas do Noroeste Company. Three samplings were necessary throughout the research work. the raw water samples characteristics relevant for this work are summarized in Table 1.
Table 1 Characteristics of raw groundwater used in the laboratory tests
The experiments were carried out in a laboratory filtration cell (Amicon type – Millipore, model 8400) with a capacity of 400 mL (Fig. 2). This was designed to work with membranes of a diameter of 76 mm.

Three different types of membranes were used – two UF membranes of polyethersulfone and one MF membrane of PAN/PVC. Before each run, each membrane was washed with distilled water and permeability of the raw water was measured.
The chemical analysis of the arsenic on the original feed as well as the resulting permeates was carried out with an Atomic Absorption Spectrophotometer (AAS) equipped with a hydride generation module (Fig. 3). The iron concentration was also measured using the same AAS apparatus.

All laboratory tests were performed keeping the same operating conditions and by adopting the same procedures. The samples were preserved adding nitric acid. The pH was measuring using a pH meter and the conductivity with a laboratory conductivimeter.
The filtration tests performed were conducted at 22o C, with a constant agitation speed of 100 rpm, applying pressures between 0.5 and 3 bar, with increments of 0.5 bar. For each of these pressure values filtration experiments with distilled water were performed to characterized the hydraulic permeability of the membranes.
In the experiments with different iron to arsenic ratios, a preliminary filtration of the solution was made with a 0.45 μm filter (Fig. 4).

Solar oxidation and removal of arsenic (SORAS) is a simple method that uses irradiation of water with sunlight in PET (or other UV-A transparent) bottles to reduce arsenic levels from drinking water. The SORAS method is based on photochemical oxidation of arsenite, As(III), followed by precipitation or filtration of arsenate, As(V), adsorbed on Fe(III) oxides (Fig. 5, Wegelin et al., 2000).
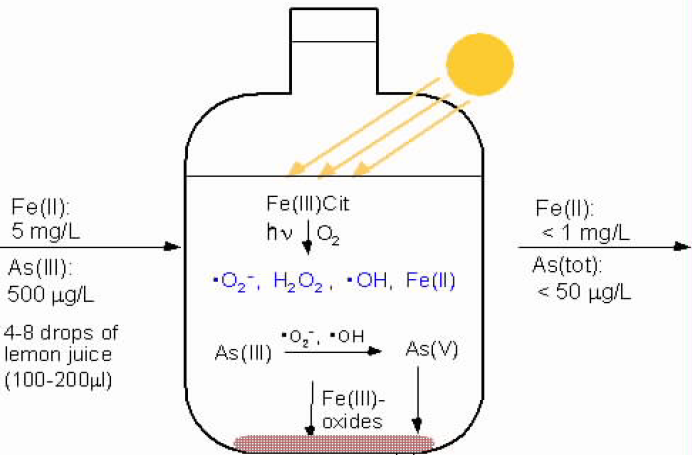
SORAS process removes arsenic in a two-step procedure. In the first step, arsenite is oxidized to the strongly adsorbing As(V). In the second step, Fe(III) (hydr)oxides formed from naturally present iron are allowed to settle to the bottom of the container with the adsorbed As(V) and the clear water is decanted(Fig. 6). Instead of adding chemical oxidants such as chlorine or permanganate, reactive oxidants are produced photochemically with sunlight.

3. RESULTS
3.1. Hydraulic permeability of membranes
Figure 7 shows the behaviour of the permeate flux variation with the pressure for the membranes used in this study and described in Table 2.
Table 2 Characteristics of membranes used in the laboratory tests
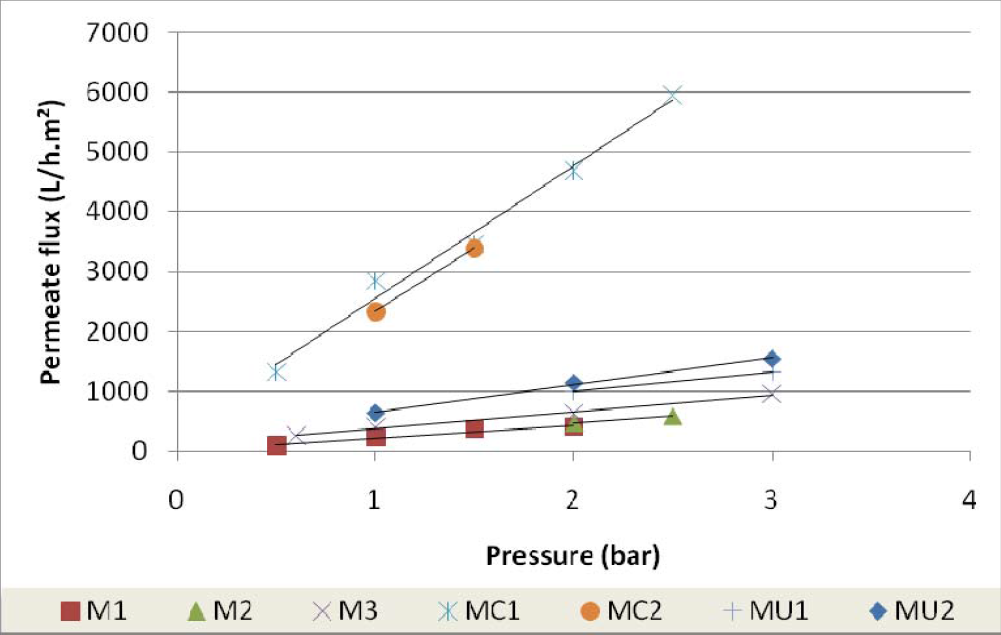
The obtained results showed that the hydraulic permeability coefficient of microfiltration membranes is approximately ten times higher than that for ultrafiltration
3.2. Efficiency of the arsenic removal by membrane filtration
For the range of pressure values tested, this operational parameter seems not to play a major role in arsenic removal efficiency (Figure 8).
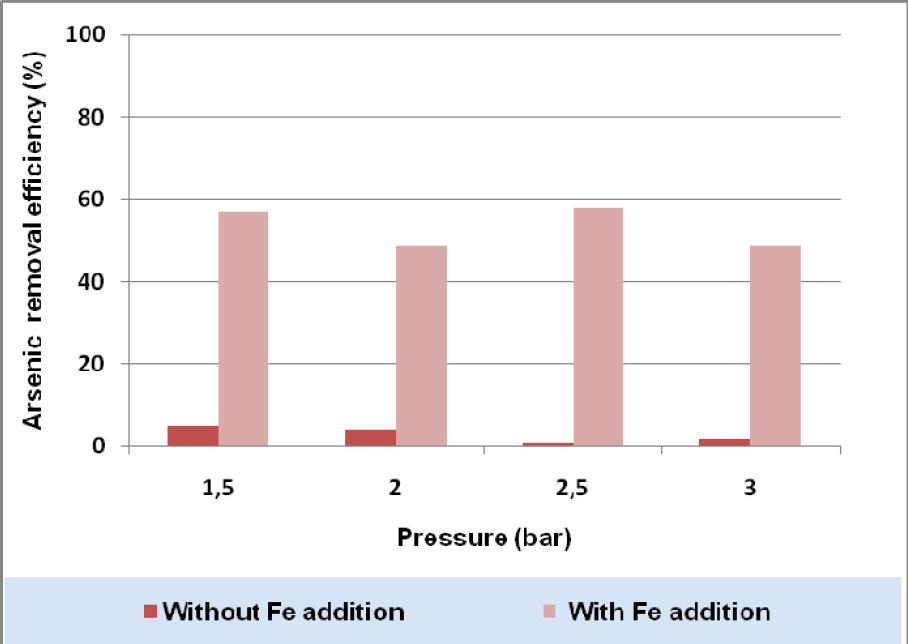
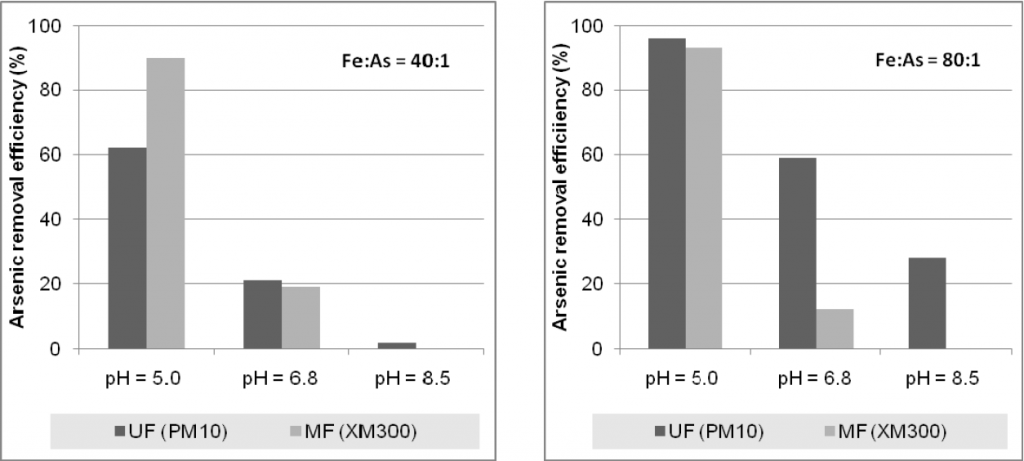
Figure 9 shows the results obtained for a raw water with a initial arsenic concentration of 15 ppb that was tested at three different pH values (5.0, 6.8 and 8.5) and two different Fe:As ratios (40:1 and 80:1). The analysis of the results allows the conclusion that the best arsenic removal efficiencies occur at pH 5 in both ultrafiltration and microfiltration processes.
To evaluate the efficiency of arsenic removal with different iron to arsenic ratios, a set of experiments was performed with feed water containing several concentrations of arsenic and iron corresponding to Fe:As ratios of 15:1, 20:1, 40:1, 80:1, 100:1 e 200:1. Feed water was prepared at neutral pH and only aeration was performed. Figure 10 shows that for higher values of Fe:As ratio (80:1, 100:1 and 200:1) higher arsenic removal efficiencies were achieved.
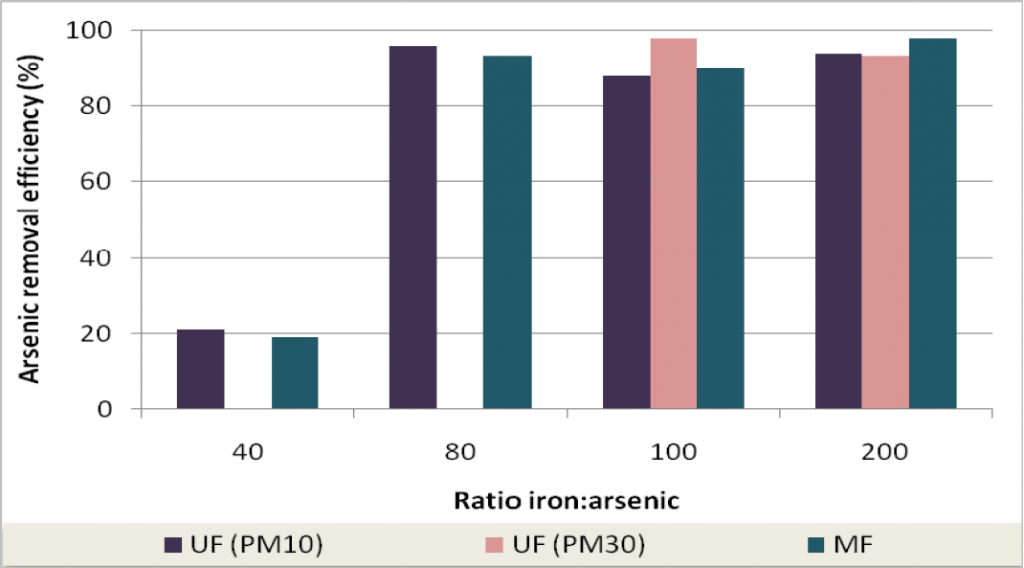
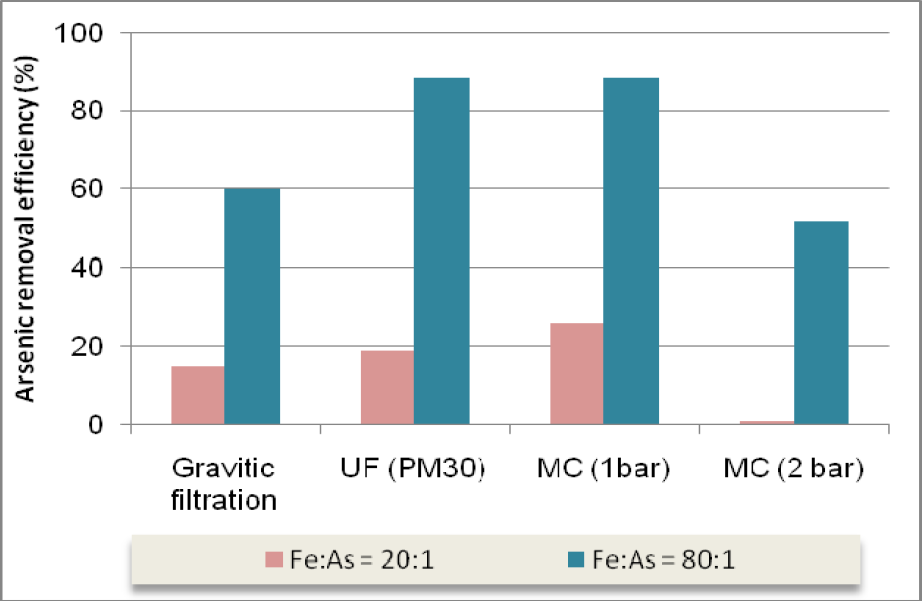
The addition of an accurate iron concentration (dependent on pH value) combined with a preliminary filtration of raw water allowed to improve the arsenic removal efficiency, even without the later utilization of membrane filtration. It shows that it is possible to remove more than 60% of arsenic for Fe:As ratios of 80:1, using gravity filtration processes only (Fig. 11). This conclusion is very important because low-rate filtration is a conventional unit process present in the most WTP, which could be improved for arsenical removal propose if the Fe:As ratio is efficiently controlled.
3.3. Arsenic removal efficiency applying SORAS technique
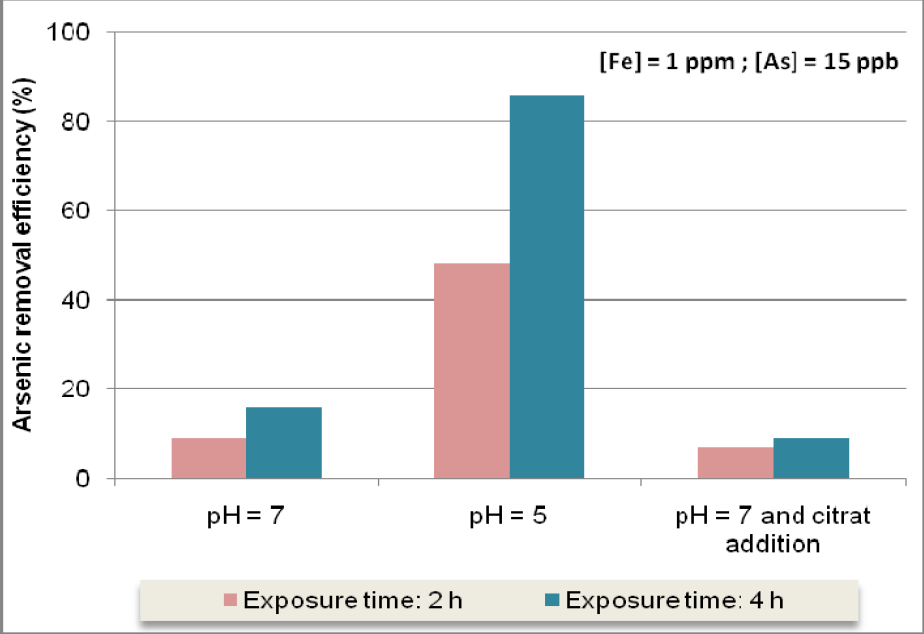
Experimental results depicted in Figure 12 show that arsenic removal efficiency using the SORAS method can achieve very interesting results for a sun exposure time of about 4 hours, when an accurate amount of iron is added to raw water, namely if pH value is low (approximately 5).
4. CONCLUSIONS
The raw water used in this study (even though from a groundwater catchment) did not present sensitive iron concentrations and made it necessary to add this constituent in the laboratory. However, this phenomenon usually does not occur in groundwater, thus it is possible to obtain the desired arsenic removal efficiencies only with iron that usually exists in the natural water sources.
The raw water used in this study (even though from a groundwater catchment) did not present sensitive iron concentrations and made it necessary to add this constituent in the laboratory. However, this phenomenon usually does not occur in groundwater, thus it is possible to obtain the desired arsenic removal efficiencies only with iron that usually exists in the natural water sources.
The experimental results obtained in this research work showed that arsenic removal efficiency using membrane separation processes increases for lower pH values (below 6) and higher Fe:As ratios (above 100:1). On the other hand, preliminary filtration or solar oxidation can be used for a sensitive removal efficiency improvement with low treatment costs increase.
The performances of ultra and microfiltration are excellent and very similar when the ideal operational conditions are presented (pH = 5-6; Fe:As ratios near 200:1) reaching a range between 93 and 98%.
The arsenic removal efficiency was not sensitive to test pressure variation. A similar conclusion had already been obtained in previous research works on micro and nanofiltration processes (Caniyilmaz, 2005; Lazarova, 2009).
The major (and unexpected) conclusion of this work is that microfiltration also led to excellent arsenic removal performance. So, the possibility of selecting membranes with higher permeate flux seems to have the potential for providing enormous gains in energy efficiency and lower construction and operational costs when applied to the real scale.
SOURCE: António A. L. S. D, Sara L. C. O, Teresa A (2010) – Arsenic Removal From Drinking Water By Advanced Filtration Processes