Electrochemical water treatment is related to the physical-chemical water treatment methods. Electrochemical treatment is characterized by multistage and relative complexity of physical and chemical phenomena occurring in electrochemical reactors (electrolyzers). The mechanism and rate of occurrence of the individual reaction steps are dependent on many factors, which have to be identified to determine the optimal reactor design and conditions for its operation. Based on the physical-chemical properties, electrochemical methods for wastewater treatment applications can be divided into three main categories. They are conversion methods, separation methods, and combined methods (Fig. 1).
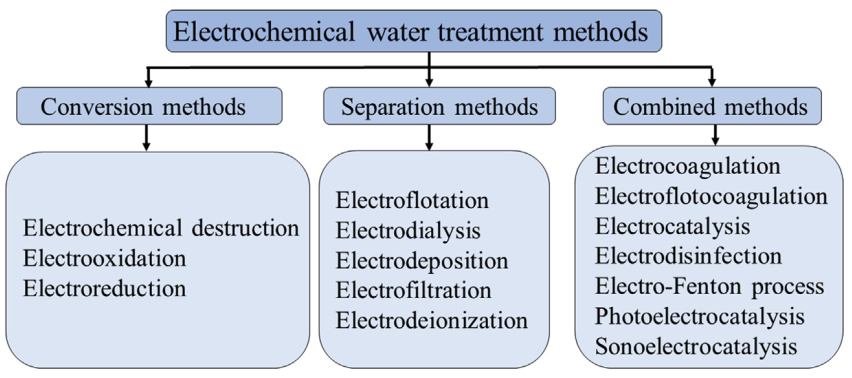
FIGURE 1. Classification of electrochemical methods by the mechanism of treatment.
The conversion method provides a change of physical-chemical and phase characteristics of dispersed pollutants in regard to their neutralization, conversion, and removal from the wastewater. The transformation of impurities can undergo a series of successive stages, starting with the electronic interaction of soluble compounds and ending with the change of electrosurface and volume characteristics of suspended substances contained in the wastewater. Electrooxidation, electroreduction, and electrocoagulation are common conversion methods. Electrooxidation and electroreduction are used for water treatment from dissolved impurities such as cyanides, thiocyanates, amines, alcohols, aldehydes, nitro compounds, azo dyes, sulfides, and thiols. Electrochemical oxidation processes lead either to complete decomposition of compounds present in the wastewater to CO2, NH3, water or formation of simple nontoxic substances, which can be removed by other methods. Different insoluble conductive materials such as graphite or mixed metal oxide electrodes (lead dioxide, manganese, ruthenium, iridium, etc., applied to a titanium substrate) are used as anodes. Cathodes are usually made of molybdenum, tungsten alloy with iron or nickel, graphite, stainless steel, and other metals and metal alloys.
Separation methods are used for pollutants concentration in the local volume of the solution without significant changes in their phase or physical-chemical properties. Separation of impurities from water is conducted mainly by electrogenerated gas bubbles in electroflotation or by the power of the electric field, which provides transport of charged particles in the water during electrodialysis.
Combined methods of electrochemical wastewater treatment incorporate one or more conversion and separation methods in one reactor. The electrocoagulation method is based on the electrolysis process using steel or aluminum anodes subjected to electrolytic dissolution. Dissolved iron or aluminum cations pass to the electrolyte solution where they react with pollutants forming flakes and causing flake precipitation. In general, during electrocoagulation pollutants lose their aggregative stability and as a result undergo sedimentation, i.e., phase separation. However, in addition to phase separation, coagulant can cause pollutant transformation. For example, dissolved Fe(II) ions reduce Cr(VI) ions to Cr(III) ions during the coagulation process.
All electrochemical processes take place at the electrodes while passing a direct electric current through the electrolyte solution. Electrochemical methods can be used for drinking water and wastewater treatment. In the case of wastewater treatment, there is a possibility to extract valuable products from water by a relatively simple technological scheme without the use of chemicals. The main disadvantage of these methods is the high energy consumption. Depending on the required effect of water treatment suitable electrochemical method and reactor can be chosen.
Electrolyzers for the electrochemical water treatment can be classified by the following features:
- Flow kinetics (continuous or batch);
- Hydrodynamic operation (pressurized or nonpressurized);
- Reactor type (open, close, diaphragm, or membrane cell);
- Movement of water in the interelectrode distance (horizontal, angled, vertical with the ascending and descending water flow);
- Type of impact on pollutants (electric field, electrode process, electric discharge or complex effects).
The effectiveness of electrochemical methods is evaluated by a number of parameters such as current density, overpotential, current efficiency, and energy consumption.
Source: S. Mika, S. Marina. Electrochemical Water Treatment Methods Fundamentals, Methods and Full Scale Applications. Butterworth-Heinemann, pages 13 – 15, 2017.