Because chemical reactions are usually associated with the rearrangement of charged particles such as electrons and atomic nuclei, chemical and electrical phenomena are closely linked. Chemical transformation occurring owing to the external applied electrical current or leading to generation of electrical current is studied in electrochemistry. Electrochemistry studies the mutual conversion of chemical and electrical forms of energy. Electrochemical reactions are of great practical importance. Example: chemical current sources such as batteries, electrolysis is used in industries, electroplating is used for the protection of steel products from corrosion, for decorative purposes. Electrochemical processes are the basis of many modern methods of analysis.
So what is the difference between chemical and electrochemical reactions? Let us consider the following chemical reaction:
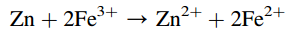
If this reaction is a chemical process, it will be characterized by some peculiarities. The chemical reaction is only possible in a case of collision of the reactants with each other. Hence, the need for contact between reacting species is the first feature of the chemical transformation. The transfer of electrons from one particle to the other or from the reducing agent (Zn) to the oxidant (Fe2+) is possible only at the time of the collision. The electron path will be very short and this is the second feature of the chemical process. Collisions can occur at any point in the reaction volume and in all relative positions of reacting species, so the electronic transitions can be performed in any direction in space. Randomness of particle collisions and electron transfer are the third feature of the chemical reaction. As a result of these peculiarities, the energy effect of the chemical reaction is expressed in the form of release or absorption of heat. It is necessary to set certain conditions in the system to convert energy from chemical reactions to electrical energy, i.e., to create an electrochemical process.
In electrochemical processes, the transfer of electrons from one reactant to another is performed over a significantly long path. It is explained by the fact that the production and consumption of electric energy is always associated with the passage of electric current, which is a stream of electrons traveling along the same path. Therefore, spatial separation of the reactants (reducing and oxidizing agents) is required for electrochemical processes to keep electrons flow from reducing to oxidizing agents. In this regard, direct contact between reactants should be replaced with the two metal plates connected to each other by a metallic conductor. To ensure the continuous passage of electric current through the reactionary space, charge carriers having a high ionic conductivity should be present or added in the reactionary solution. Thus, a system called an electrochemical cell is required to conduct electrochemical reactions. Thus, a system called an electrochemical cell is required to conduct electrochemical reactions.
Wastewater treatment by electrochemical methods is based on conducting the electrolysis process. To conduct the electrolysis an external source of electrical energy is required to generate and maintain a proper potential and as a result electrochemical reactions at anode and cathode, which are placed into the electrochemical cells (for example, into industrial electrolyzer). Michael Faraday was the first scientist who investigated the relationship between the amount of electric charge Q (current I multiplied by time t) passed through the electrode/electrolyte solution interface and chemical reactions caused by this charge. In 1832 Faraday reported that the amount of electricity required to produce a given quantity of substances does not depend on the electrode size, number of working electrodes, and the distance between electrodes. It was stated that the mass m of the substance liberated at an electrode is directly proportional to the electric charge Q, passed through the electrolyte and directly proportional to the equivalent weight (M/z) of the element for a given amount of electricity:
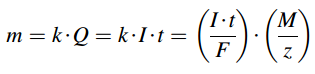
where k is electrochemical equivalent of a substance, k = M/(F×z), M is molar mass of a substance, 1 F = 1 mol×e– = 6.02×1023×e– = e×NA = 26.8 A.h/mol = 96,485.33289(59) C/mol is Faraday constant, z is the number of electrons participating in the reaction (valency of ion of the substance).
Michael Faraday together with his friend William Whewell developed a new terminology in electrochemistry. He called conductors immersed in the solution such as the electrodes (earlier they were called poles), introduced the concept of electrolysis (chemical changes associated with the current passage), electrolyte (conductive liquid in electrochemical cells), anode (electrode with oxidation reaction on it), and cathode (an electrode with the reduction reaction on it). The charge carriers in liquids were called ions (from the Greek wanderer); the ions moving to the anode (positive electrode) were called anions and to the cathode (negative electrode) cations. Colloids and suspended solids also can participate in the transfer of electric current; however, due to low mobility, they can carry only an insignificant part of electric current.
Electrochemical equivalent can be used to calculate the amount of reactive substance in the anodic and cathodic processes, such as anodic dissolution of metal, gas evolution at the cathode, and products of EO. Electrochemical equivalent value for the same substance may differ depending on the electrochemical process, in which the substance participates. Let us consider three different reactions of chlorine, hypochlorite, and chlorate electrolytic evolution and anode’s half-reactions:
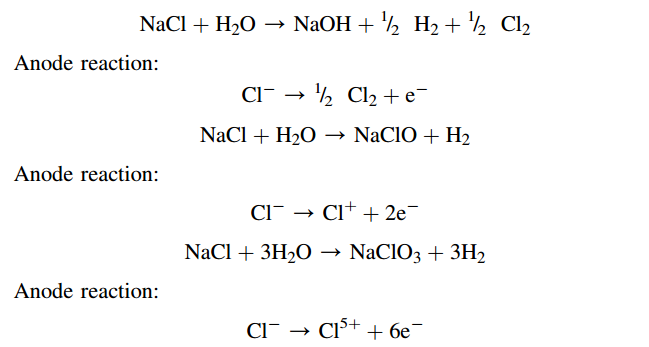
As can be seen from anode’s reaction the number of electrons participating in the electrolytic formation of chlorine, hypochlorite, and chlorate are equal to 1, 2, and 6 electrons, which means that z = 1, 2, or 6, respectively. Thus, electrochemical equivalents of NaCl for chlorine, hypochlorite, and chlorate formation are equal to z = 58.44/(1×96,485) = 6.1×104 g/C = 0.61 mg/C; 0.3 mg/C; and 0.1 mg/C, respectively.
Faraday’s laws are strictly observed. Observed deviations from Faraday’s laws often associated with the presence of unaccounted parallel electrochemical reactions, such as oxygen evolution reactions, hydrogen peroxide formation, or product recombination reactions. Deviations from Faraday’s law in industrial systems are associated with Faradic current losses appearing as heat or unwanted by-products, loss of material by spraying the solution, etc. The ratio of the actual amount of product (charge/electrons) obtained/spent in electrolysis to the theoretical amount of product (charge/electrons) calculated based on Faraday’s law is typically below one in the technological processes. This relation is called current efficiency (CE; faradaic or coulombic efficiency):
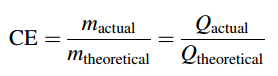
Current efficiency in EO (Electro-oxidation ) processes of organic pollutants can be monitored through the COD (Chemical Oxygen Demand) decay values at a constant current using Eq.
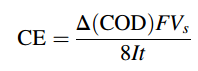
where ΔCOD is the COD decrease during degradation of pollutants at time t and 8 is the oxygen equivalent mass (q equiv-1 ).
J. Gibbs and W. Nernst contributed to the development of electrochemical thermodynamics and particularly to the determination of the nature of electrical potential (voltage) in the electrochemical cell and the balance between electrical, chemical, and thermal energies. The electrochemical potential is determined by the energy of the chemical processes occurring in the electrochemical cells and also depends on their kinetics.
Source: S. Mika, S. Marina. Electrochemical Water Treatment Methods Fundamentals, Methods and Full Scale Applications. Butterworth-Heinemann, pages 15 – 18, 2017.